SimChat | Q&A with Authors Michael J. Moore, PhD, and Wesley Anderson, PhD, Highlighting Their Publication Utilizing our NerveSim® Platform to Research Opioid Treatment Alternatives
News and Blog
We are proud to announce our published paper: Morphine-sensitive synaptic transmission emerges in embryonic rat microphysiological model of lower afferent nociceptive signaling in Science Advances.
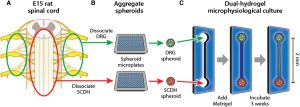
The work is a collaboration between the academic lab of AxoSim’s Chief Science Officer, Michael J. Moore, Ph.D., at Tulane University, and our lab, co-authored by AxoSim’s Research Scientist Wesley Anderson, Ph.D. The science team built a 3D tissue model of the pain synapse (Dorsal Root Ganglion (DRG) to dorsal horn (DH)) and demonstrated responses to lidocaine and morphine along with other drugs known to affect this synapse location. Michael and Wesley took some time to answer questions and dive deeper into the publication for our blog.
What would you say is the biggest takeaway from your recent publication?
Wesley: After creating a 3D in-vitro model of the DRG to DH synapse site, which can be used for screening therapeutics. We confirmed functional synapses through IHC and electrical stimulation.
Is there a reason you decided to focus on pain alternatives?
Michael: Our society has been experiencing a calamitous epidemic of addiction to synthetic opioids. Because of this, we wanted to see if the NerveSim® platform could be adapted to address this terrible problem and find alternative non-addictive pain therapies.
What is it about the spinal cord DRG and DH that makes it a good target in your research?
Wesley: The site where the DRG synapses onto the DH is a great target for pain research because this is the location where primary pain information is transmitted from the sensory neurons to the CNS on the way to the brain. Opioids have analgesic effects at this location, which makes it the perfect site to start studying the actions of these drugs and alternatives in-vitro.
Why do you think the stiffness of your culture gels had such an effect on growth and electrophysiology?
Michael: We were working with two different cell types–one found in the peripheral nerve and one found in the central nervous system–and we knew from previous research that these cell types would likely respond to their microenvironment differently. Compared to other tissues, neurons tend to survive and remain healthier in softer rather than stiffer environments. However, some types of neurons, such as peripheral nerves, may actually grow more quickly in stiffer environments. Due to this, we needed to find gel types whose stiffness and other physical and chemical properties would encourage the growth of peripheral nerve cells toward spinal cord cells while preventing growth in the opposite direction. It was extremely difficult to find such a balance. Interestingly, once we found a gel type that encouraged the growth of both cell types, the spinal cord cells spontaneously avoided growing toward the peripheral nerve cells. It was as if the cells’ programming told them not to go in that direction. This was surprising and awe-inspiring.
What is the benefit of using these two culture types together instead of running the studies in separate cultures in parallel?
Wesley: We have used these two culture systems because it best represents the system in our bodies. The closer we can replicate these in-vivo structures, the more likely we are to discover therapeutics that can be used clinically.
Where do you see taking this study next? Are you planning to expand your design or begin running test articles through the platform?
Michael: We are currently working on making the same kind of model system with human cells that are derived from stem cells. Additionally, we are including critically important support cells (e.g. astrocytes and microglia), which are actually part of the immune system. These cells are thought to play a role in the development of chronic pain, thus including them may allow us to model this process and find more effective treatments for chronic pain. These cells are also implicated in the development of tolerance–the need for more of a drug to get the same effect–which is a key step in the process toward addiction. We may be able to model certain biological mechanisms that lead to tolerance, which could be useful for finding drugs that have less addiction potential.